⚡ Quick Summary
This study presents a novel approach for the automatic segmentation of clear cell renal cell carcinoma (ccRCC) using deep learning techniques, achieving a remarkable classification accuracy of 96.67%. The research also explores the tumor microenvironment (TME), revealing significant correlations between TME characteristics and immunotherapy responses.
🔍 Key Details
- 📊 Methodology: Automatic segmentation using deep learning and convolutional neural networks.
- 🧩 Imaging Technique: Whole-slide imaging (WSI) for high-resolution tissue examination.
- ⚙️ Analysis: Inverse threshold segmentation for lymphocyte and collagen fiber distribution.
- 🏆 Performance Metrics: 96.67% classification accuracy, 94.29% sensitivity.
🔑 Key Takeaways
- 🤖 AI Technology: Deep learning enhances the precision of ccRCC diagnosis.
- 🌱 TME Insights: High infiltration of CD3+ and CD8+ T cells correlates with advanced tumor stages.
- 📈 Collagen Proliferation: Linked to tumor growth and metastasis.
- 🔬 Immunotherapy Optimization: Understanding TME can improve patient-specific treatment strategies.
- 🌍 Clinical Relevance: Findings may guide future immunotherapeutic approaches for ccRCC.
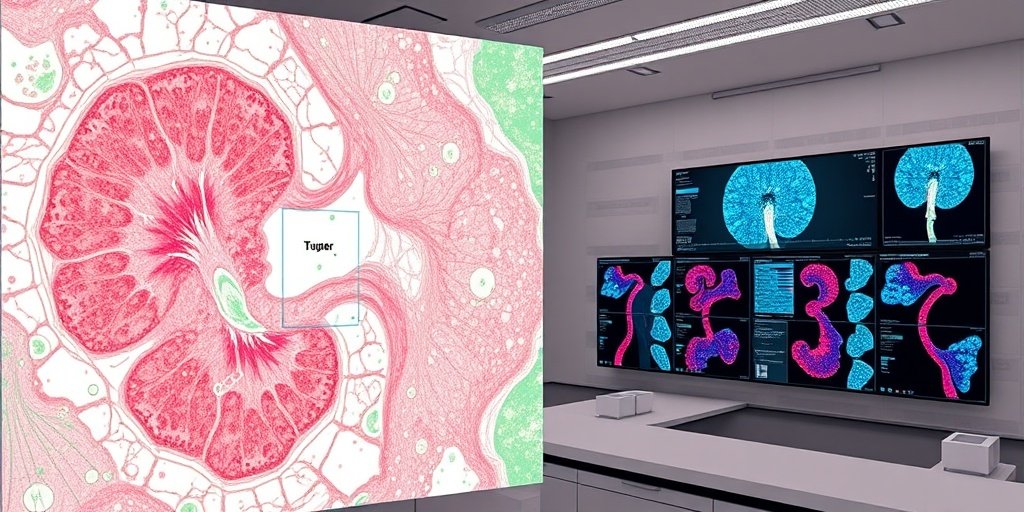
📚 Background
Clear cell renal cell carcinoma (ccRCC) is a prevalent form of kidney cancer, and its diagnosis has been significantly enhanced by whole-slide imaging (WSI). This advanced imaging technique allows for detailed examination of tissue sections, which is crucial for accurate diagnosis and effective management of renal cancers. The tumor microenvironment (TME) plays a vital role in influencing therapeutic outcomes, particularly in the context of immunotherapy, making it essential to understand the interactions between cancer cells and their surrounding environment.
🗒️ Study
The study aimed to develop a novel method for the automatic segmentation of ccRCC regions using deep learning techniques. By employing advanced convolutional neural networks, researchers were able to distinguish tumor areas from surrounding tissues effectively. Additionally, the study utilized inverse threshold segmentation to quantitatively analyze the distribution of lymphocytes and collagen fibers in immunohistochemical and Masson’s trichrome-stained images, thereby enhancing the precision of histopathological assessments.
📈 Results
The deep learning model demonstrated impressive performance, achieving a classification accuracy of 96.67% on image patches and a sensitivity of 94.29%. The analysis of TME characteristics revealed that a high infiltration of CD3+ T cells, particularly CD8+ cytotoxic T cells, was more prevalent in patients with advanced-stage tumors. Furthermore, the proliferation of collagen fibers was significantly correlated with tumor growth and metastasis, highlighting the importance of TME in ccRCC progression.
🌍 Impact and Implications
The findings from this study underscore the potential of artificial intelligence (AI) to revolutionize the diagnosis and treatment of ccRCC. By integrating deep learning techniques into tumor segmentation and TME analysis, researchers can gain valuable insights that may guide immunotherapy strategies. This approach not only streamlines the diagnostic process but also enhances the understanding of tumor biology, ultimately aiming to improve therapeutic outcomes and patient survival rates.
🔮 Conclusion
This study highlights the transformative potential of AI in the field of oncology, particularly in the context of ccRCC. By applying deep learning to tumor segmentation and TME analysis, we can pave the way for more personalized and effective immunotherapeutic strategies. Future research should focus on integrating these findings into clinical practice to optimize treatment protocols and enhance patient care.
💬 Your comments
What are your thoughts on the integration of AI in cancer diagnosis and treatment? We would love to hear your insights! 💬 Leave your comments below or connect with us on social media:
Automatic segmentation of clear cell renal cell carcinoma based on deep learning and a preliminary exploration of the tumor microenvironment.
Abstract
BACKGROUND: Whole-slide imaging (WSI) is increasingly becoming a standard method for diagnosing clear cell renal cell carcinoma (ccRCC). This advanced imaging technique allows for high-resolution examination of tissue sections, improving diagnosis and management of renal cancers. Immunotherapy has emerged as an effective treatment for tumors; however, the differential characteristics of the tumor microenvironment (TME) significantly influence therapeutic outcomes. Understanding the interactions between cancer cells and the TME is essential for optimizing immunotherapeutic strategies. This study aims to investigate the characteristics of the TME in ccRCC using WSI, with the goal of identifying factors that might influence immunotherapy response and improving therapeutic strategies.
METHODS: In this study, we proposed a novel method for the automatic segmentation of ccRCC regions based on deep-learning techniques. This method uses advanced convolutional neural networks to effectively distinguish between tumor areas (TAs) and surrounding tissues. Additionally, we employed inverse threshold segmentation to quantitatively analyze the results and spatial distributions of lymphocytes and collagen fibers in immunohistochemical and Masson’s trichrome-stained images. This comprehensive approach not only streamlines the diagnostic process but also enhances the precision of histopathological assessments.
RESULTS: Our model had a classification accuracy of 96.67% on image patches and a sensitivity of 94.29%, demonstrating its ability to segment TAs both accurately and efficiently. The distribution of cluster of differentiation (CD)3+ and CD8+ T lymphocytes, and collagen fibers in patients at different tumor-node-metastasis (TNM) stages was analyzed. The results revealed that a high infiltration of CD3+ T cells, particularly CD8+ cytotoxic T cells, was more prevalent in patients with advanced-stage tumors. Additionally, the proliferation of collagen fibers in tumors was found to be significantly correlated with tumor growth and metastasis.
CONCLUSIONS: Our results underscore the potential of artificial intelligence (AI) technology to provide novel insights to guide ccRCC immunotherapy. By applying deep learning to tumor segmentation and TME analysis, this methodology offers a promising approach to improve the understanding of tumor biology and therapeutic outcomes. Future research should focus on integrating these findings into clinical practice to optimize patient-specific immunotherapeutic strategies, and thus advance treatment protocols and improve the survival rates of ccRCC patients.
Author: [‘Tang H’, ‘Zhao H’, ‘Yu S’, ‘Wang Y’, ‘Su J’, ‘Wang X’, ‘Schmeusser BN’, ‘Zapała Ł’, ‘Yu G’, ‘Feng N’]
Journal: Transl Androl Urol
Citation: Tang H, et al. Automatic segmentation of clear cell renal cell carcinoma based on deep learning and a preliminary exploration of the tumor microenvironment. Automatic segmentation of clear cell renal cell carcinoma based on deep learning and a preliminary exploration of the tumor microenvironment. 2025; 14:2059-2074. doi: 10.21037/tau-2025-400